| 原産地: | 中国(本土) | 銘柄: | Bosom/homescan/oem | モデル番号: | 1h0652 | タイプ: | 病理学の分析装置 | 標本: | 全血、 血清、 プラズマ | 形式: | パネルカード | 証明書: | iso13485、 fsc | hbv: | hbs抗原、 hbs抗体、 抗原、 抗原、 hbcab |
包装
| 包装: | テスト1/ポーチ、 25のテスト/kitまたは40テスト/kit。 |
仕様
Rapid HBV Hepatitis B Test Card 5 in 1
FOR THE QUALITATIVE ASSESSMENT OF THE MARKERS OF HEPATITIS B VIRUS INFECTION IN HUMAN SERUM, PLASMA OR WHOLE BLOOD
For In Vitro Diagnostic Use Only
1H10C2, HBV-3 Panel Test
1H0652, HBV-5 Panel Test
1H11C2, HBV-2 Panel Test
INTENDED USE
Rapid HBV-2, HBV-3, and HBV-5 Panel Test, hereinafter referred to HBV Panel Test, is a rapid immunochromatography assay for the qualitative detection of the markers of Hepatitis B virus including Hepatitis B virus surface antigen (HBsAg), Hepatitis B virus surface antibody (anti-HBs/HBsAb), Hepatatis B virus envelope antigen (HBeAg), Hepatatis B virus envelope antibody (HBeAb/anti-HBe) and Hepatatis B virus core antibody (anti-HBc/HBcAb) in human serum, plasma or whole blood specimens. It is intended for use in medical institution as an aid for diagnosis and management of patients related to infection with hepatitis B as well for screening of blood donors or blood products.
SUMMARY
Hepatitis B virus (HBV) is an enveloped; double- stranded DNA virus belonging to the Hepadnaviridae family and is recognized as the major cause of blood transmitted hepatitis together with hepatitis C virus (HCV). Infection with HBV induces acute or chronic liver diseases and in some cases that can lead to cirrhosis and carcinoma of the liver. Hepatitis B surface antigen or HBsAg, which was previously described as Australia antigen is the most important protein of the envelope of Hepatitis B Virus. The surface antigen contains the determinant “a”, common to all known viral subtypes, immunologically distinguished in two distinct subgroups (ay and ad). HBV has 10 major serotypes and four HBsAg subtypes have been recognized (adw, ady, ayw, and ayr). HBsAg can be detected 2 to 4 weeks before the ALT levels become abnormal and 3 to 5 weeks before symptoms develop.
TEST PRINCIPLE
HBsAg test is a double antibody sandwich immunoassay. Colloidal gold conjugated anti-HBsAg antibody complexes are dry-immobilized in the test device. When the sample is added, it migrates by capillary diffusion through the strip rehydrating the gold conjugate complexes. If present, HBsAg will react with the gold conjugate complexes forming particles. These particles will continue to migrate along the strip until the Test Zone (T) wher they are captured by anti-HBsAg antibodies immobilized there and a visible red line appears. If there is no HBsAg in sample, no red line will appear in the Test Zone (T). The gold conjugate complexes will continue to migrate alone until they are captured in the Control Zone (C) by immobilized goat anti-mouse IgG antibody aggregating a red line, which indicates the validity of the test.
HBsAb test is a double antigen sandwich immunoassay. Colloidal gold conjugated HBsAg complexes are dry-immobilized in the test device. When the sample is added, it migrates by capillary diffusion through the strip rehydrating the gold conjugate complexes. If present, HBsAb will react with the gold conjugate complexes forming particles. These particles will continue to migrate along the strip until the Test Zone (T) wher they are captured by HBsAg immobilized there and a visible red line appears. If there is no HBsAb in sample, no red line will appear in the Test Zone (T). The gold conjugate complexes will continue to migrate alone until they are captured in the Control Zone (C) by immobilized goat anti-HBsAg antibody aggregating a red line, which indicates the validity of the test.
HBeAg test is a double antibody sandwich immunoassay. Colloidal gold conjugated anti-HBeAg antibody complexes are dry-immobilized in the test device. When the sample is added, it migrates by capillary diffusion through the strip rehydrating the gold conjugate complexes. If present, HBeAg will react with the gold conjugate complexes forming particles. These particles will continue to migrate along the strip until the Test Zone (T) wher they are captured by anti-HBeAg antibodies immobilized there and a visible red line appears. If there is no HBeAg in sample, no red line will appear in the Test Zone (T). The gold conjugate complexes will continue to migrate alone until they are captured in the Control Zone (C) by immobilized goat anti-mouse IgG antibody aggregating a red line, which indicates the validity of the test.
HBeAb test is a competitive immunoassay. Colloidal gold conjugated anti-HBeAg antibody complexes are dry-immobilized in the test device. When the specimen is added, it migrates with the gold conjugate complexes by capillary diffusion through the strip. If present, HBeAb will compete with gold conjugate complexes for the limited amount HBeAg immobilized in the Test Zone (T). It will prevent the gold conjugate complexes from reacting with HBeAg and no red line appears in the Test Zone (T). If there is no HBeAb in the specimens, gold conjugate complexes will react with HBeAg and a visible red line appears. To serve as a procedural control, a red line will always appears in the Control Zone (C) which indicates the validity of the test.
HBcAb test is a competitive immunoassay. Colloidal gold conjugated anti-HBcAg antibody complexes are dry-immobilized in the test device. When the specimen is added, it migrates with the gold conjugate complexes by capillary diffusion through the strip. If present, HBcAb will compete with gold conjugate complexes for the limited amount HBcAg immobilized in the Test Zone (T). It will prevent the gold conjugate complexes from reacting with HBcAg and no red line appears in the Test Zone (T). If there is no HBcAb in the specimens, gold conjugate complexes will react with HBcAg and a visible red line appears. To serve as a procedural control, a red line will always appears in the Control Zone (C) which indicates the validity of the test.
MATERIAL PROVIDED
1. Rapid HBV Panel Test
2. Instructions for use
3. Disposable transfer pipet
MATERIALS REQUIRED BUT NOT SUPPLIED
1. Whole blood or plasma: Vacutainer tube, or other appropriate tube, containing heparin or EDTA as an anticoagulant
2. Serum: Vacutainer tube, or other appropriate tube, without anticoagulant
3. Timer or clock
STORAGE
Store the test device at 4 to 30oC. Do Not Freeze.
PRECAUTIONS
1. For in vitro diagnostic use only.
2. Do not use product beyond the expiration date.
3. Handle all specimens as potentially infectious.
SPECIMEN COLLECTION AND PREPARATION
1. The serum, plasma or whole blood specimen should be collected under standard laboratory conditions.
2. Heat inactivation of specimens, which may cause hemolysis and protein denaturation, should be avoided.
3. Patient samples performed best when tested immediately after collection. If specimans are to be stored, the red blood cells should be removed to avoid hemolysis. If the sample cannot be tested within 24 hours, serum or plasma should be frozen until the test can be performed. Whole blood samples should be refrigerated at 2–8oC in stead of being frozen. Allow sample to reach room temperature before proceeding.
4. Sodium azide can be added as a preservative up to 0.1% without affecting the test results.
PROCEDURE
1. Bring all materials and specimens to room temperature.
2. Remove the test device from the sealed foil pouch.
3. Place the test device on a flat horizontal surface and label the test device with specimen identity.
4 Using the transfer pipet to draw up the sample, dispense 2 drops (80-100 μl) of sample in a vertical position into the
each sample well individually.
5. Read the result at 20 minutes after adding the sample.
Note: Results read after 30 minutes may not be accurate.
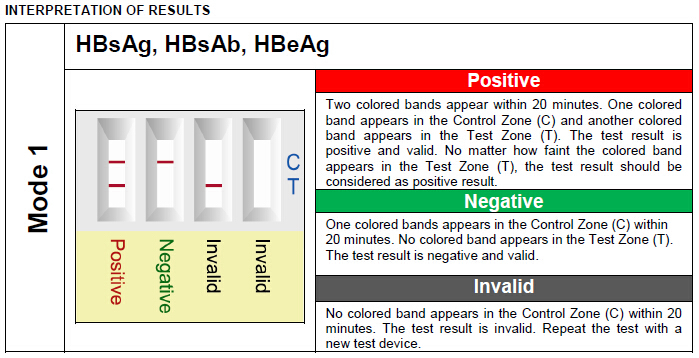
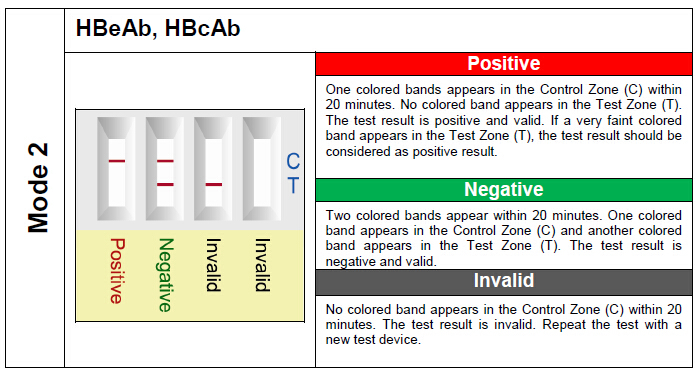

PERFORMANCE CHARACTERISTICS:
In a clinical evaluation of the performance of Rapid HBV Panel Test, using 1249 ELISA confirmed samples, the
positive agreement, negative agreement and the overall accuracy of each HBV analyte are summarized as the table.
Item Positive Agreement (%) Negative Agreement (%) Accuracy (%)
HBsAg 99.6 (467/469) 99.6 (777/780) 99.6 (1244/1249)
Anti-HBs 97.6 (522/535) 98.2 (701/714) 97.9 (1223/1249)
Anti-HBc 97.1 (642/661) 97.8 (575/588) 97.4 (1217/1249)
HBeAg 99.4 (159/160) 99.6 (1085/1089) 99.6 (1244/1249)
Anti-HBe 96.9 (372/384) 98.8 (855/865) 98.2 (1227/1249)
LIMITATIONS
1. Negative results do not rule out the possibility of hepatitis B virus exposure or infection. Infection through recent
exposure to HBV may not be detectable.
2. A test giving an invalid result should be repeated.
3. HBV panel test is a qualitative assay. The line intensity cannot be assumed to correlate with quantitative levels.